オーストラリアでのHM201の第Ⅰ相臨床試験が終了しました。
HIMUKA AM AUSTRALIA ANNOUNCES COMPLETION OF PHASE 1 CLINICAL TRIAL OF HM201 IN AUSTRALIA
~開発品HM201での最初のヒト投与試験を終了し、まもなくデータを受領~
当社のオーストラリア子会社Himuka AM Australia Pty. Ltd. が、開発品「HM201」の第I相臨床試験の終了報告をオーストラリア薬品・医薬品行政局(Therapeutic Goods Administration)に提出したことを発表しました。HM201は投与の利便性を格段に向上させた新薬候補品です。
■開発品HM201
HM201の主成分は、当社の共同創業者である北村和雄(宮崎大学特別教授)が発見したペプチド「アドレノメデュリン(AM)」をベースに新規開発されました。
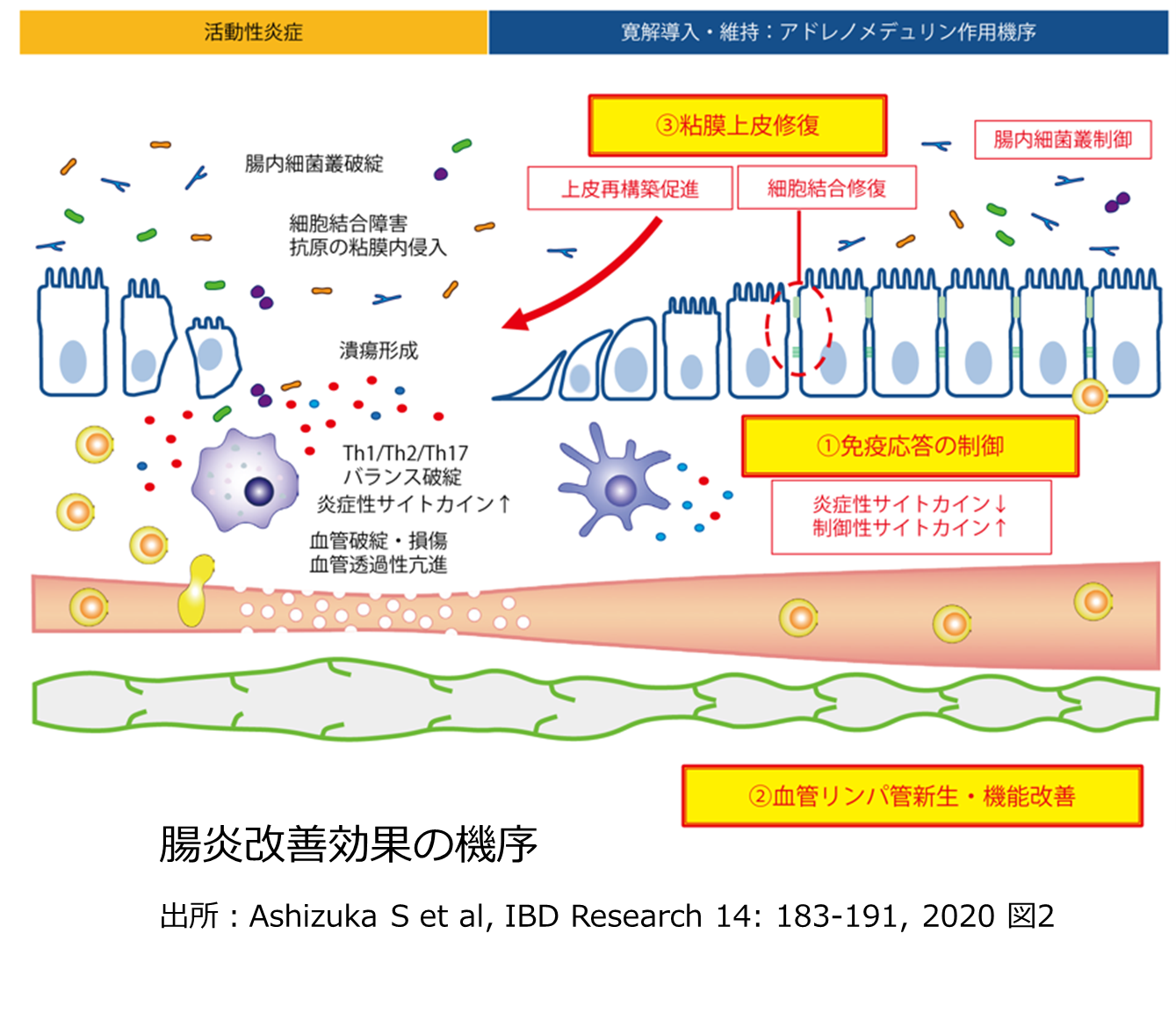
AMは上皮細胞のバリア機能の修復・維持に関わる物質です。慢性炎症により傷んだ腸粘膜の修復を促進します(下図参照)。また、AMは、抗炎症・免疫抑制を主作用とする医薬品とはたらき(機序)が異なるため、副作用リスクが低い特長も備えています。
HM201は、AMの投与における利便性を大幅に向上させることを目的に開発されました。
■臨床試験
今回の臨床試験は、オーストラリアにおいて、第I相臨床試験として実施されました。健常な方を被験者として、HM201を単回又は複数回投与し、HM201の安全性や薬物動態等を確認することを目的とする試験です。結果速報では評価項⽬に関して良好なデータが得られており、数ヶ⽉以内に最終報告を受領する予定です。
リンク:プレスリリース(PDF)
Himuka AM Australia Pty Ltd. today announced that a Phase 1 clinical trial of HM201, a novel adrenomedullin based peptide drug candidate developed for the treatment of Inflammatory bowel disease (IBD), has been completed. The preliminary results of this study demonstrated excellent data with final results scheduled to be reported in several months.
“The trial was affected by the COVID-19 pandemic and the severe flooding in Brisbane, although we overcame the difficulties with the cooperation of the subjects, the clinical site and the CRO. We are proud to have been able to submit a Clinical Trial Notification of study completion to the Therapeutic Goods Administration,” said Hiroshi Shinjo, Chief Executive Officer of Himuka AM Australia.
The Phase 1 clinical trial of HM201 was a double-blind, placebo-controlled, ascending dose, multi-cohort study in healthy subjects consisting of single ascending dose and multiple ascending dose cohorts. The study aimed to assess the safety, tolerability, and pharmacokinetics of single and multiple intravenous administrations of HM201.
(ClinicalTrials.gov Identifier : NCT05088369)
About HM201
HM201 is a novel peptide drug candidate created by improving the bioactive peptide adrenomedullin.
Adrenomedullin contributes to the regulation of homeostasis in the human body through the action of regeneration of epithelial and endothelial cells, angiogenesis, and anti-inflammation. In the Phase 2a clinical trial of adrenomedullin for steroid-resistant ulcerative colitis (UC) conducted by University of Miyazaki, Japan, they observed the complete remission at 8 weeks in patients with steroid-resistant UC receiving a certain dose of adrenomedullin.
Himuka believes that HM201 may have the same pharmacological effects as adrenomedullin and is developing HM201 with the belief that it may contribute to the healing of the intestinal mucosa in suffering patients.
About Himuka
Himuka AM Australia Pty Ltd. is an Australian company which was established by Himuka AM Pharma Corp. for the development of HM201 in Australia.
Himuka AM Pharma Corp. is a multi-asset clinical-stage biopharmaceutical company focused on developing novel treatments in areas of unmet need including refractory Inflammatory bowel disease.
Regarding Himuka’s first candidate, adrenomedullin (HM101), several Phase 2a investigator-initiated clinical trials have been conducted under programs of the Japan Agency for Medical Research and Development.
Himuka is also advancing HM201, its novel peptide drug candidate for the treatment of diseases through the improvement of the barrier function of epithelial or endothelial cells, and the function of angiogenesis.
For more information, visit https://www.himuka-am.com/
Forward-Looking Statements
This press release may contain forward-looking statements.
Statements contained in this press release concerning plans, predictions, and strategies to improve future performance (“Forward-Looking Statements”) are based on information currently available to Himuka’s management, and inevitably involve a certain element of risk and uncertainties.
Actual results may therefore differ from those in the Forward-Looking Statements. Therefore, forward-looking statements should not be relied upon as representing Himuka’s views as of any date after the date of this press release.
