オーストラリアでのHM201の第Ⅰ相臨床試験を開始しました。
HIMUKA AM AUSTRALIA ANNOUNCES INITIATION FOR PHASE 1 CLINICAL TRIAL OF HM201 IN AUSTRALIA
当社のオーストラリア子会社Himuka AM Australia Pty. Ltd. が、開発品「HM201」の最初のヒト投与試験(First-in-Human試験)をオーストラリアで開始しました。
■開発品HM201
HM201の主成分は、当社の共同創業者である北村和雄(宮崎大学特別教授)が発見したペプチド「アドレノメデュリン(AM)」をベースに新規開発されました。
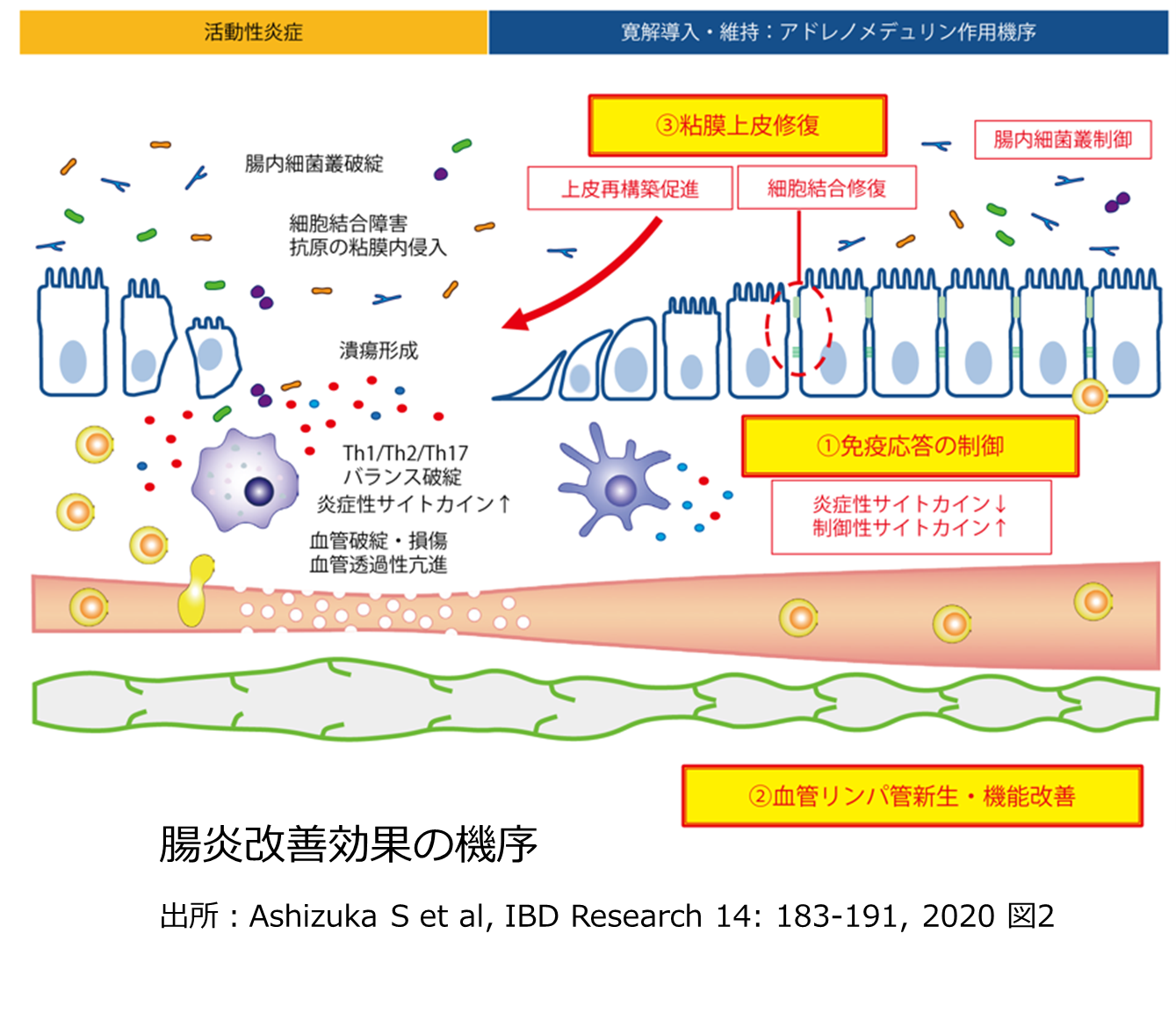
AMは上皮細胞のバリア機能の修復・維持に関わる物質です。慢性炎症により傷んだ腸粘膜の修復を促進します(下図参照)。また、AMは、抗炎症・免疫抑制を主作用とする医薬品とはたらき(機序)が異なるため、副作用リスクが低い特長も備えています。
HM201は、AMの投与における利便性を大幅に向上させることを目的に開発されました。
■臨床試験
このたびの臨床試験は、オーストラリアにおいて、第I相臨床試験として実施されます。健常な方を被験者として、HM201を単回又は複数回で投与し、HM201の安全性や薬物動態等を確認することを目的としています。
リンク:プレスリリース(PDF)
Himuka AM Australia Pty Ltd. today announced that it initiated a Phase 1 clinical trial of HM201, a novel adrenomedullin based peptide drug candidate developed for the treatment of Inflammatory bowel disease (IBD).
This clinical trial is being performed at the clinical site operated by Nucleus Network in Queensland, Australia.
“HM201 is a novel peptide drug candidate that has the potential to address the unmet need of mucosal healing in IBD patients. We are very excited about this first-in-human study as it is the first step in confirming the improvements we have made to adrenomedullin,” said Hiroshi Shinjo, Chief Executive Officer of Himuka AM Australia. “In Australia we have access to an excellent drug development environment to conduct clinical trials and we would like to express our gratitude to the many people who have and are continuing to make this trial possible.”
The Phase 1 clinical trial of HM201 is a double-blind, placebo-controlled, ascending dose, multi-cohort study in healthy subjects consisting of single ascending dose and multiple ascending dose cohorts. Himuka expects to receive the results from the Phase 1 clinical trial in the fourth quarter of 2022.
About HM201
HM201 is a novel peptide drug candidate created by improving the bioactive peptide adrenomedullin.
Adrenomedullin contributes to the regulation of homeostasis in the human body through the action of regeneration of epithelial and endothelial cells, angiogenesis, and anti-inflammation. In the Phase 2a clinical trial of adrenomedullin for steroid-resistant ulcerative colitis (UC) conducted by University of Miyazaki, Japan, they observed the complete remission at 8 weeks in patients with steroid-resistant UC receiving a certain dose of adrenomedullin.
Himuka believes that HM201 has the same pharmacological effects as adrenomedullin and is developing HM201 with the belief that it will contribute to the healing of the intestinal mucosa in suffering patients.
About Himuka
Himuka AM Australia Pty Ltd. is an Australian company which is established by Himuka AM Pharma Corp. for the development of HM201 in Australia.
Himuka AM Pharma Corp. is a multi-asset clinical-stage biopharmaceutical company focused on developing novel treatments in unmet need areas including refractory Inflammatory bowel disease.
Regarding Himuka’s first candidate, adrenomedullin (HM101), several Phase 2a investigator-initiated clinical trials have been conducted under the programs of the Japan Agency for Medical Research and Development.
Himuka is also advancing HM201, its novel peptide drug candidate for the treatment of diseases through the improvement of the barrier function of epithelial or endothelial cells, and the function of angiogenesis.
Himuka AM Pharma Corp. is selected to be part of the J-Startup program by Japan External Trade Organization (JETRO). JETRO’s mentorship and business support helps promote Himuka’s projects to an international audience.
Forward-Looking Statements
This press release may contain forward-looking statements.
Statements contained in this press release concerning plans, predictions, and strategies to improve future performance (“Forward-Looking Statements”) are based on information currently available to Himuka’s management, and inevitably involve a certain element of risk and uncertainties.
Actual results may therefore differ from those in the Forward-Looking Statements. Therefore, forward-looking statements should not be relied upon as representing Himuka’s views as of any date after the date of this press release.
